Grade 8 Science : Properties of Matter - Part 5
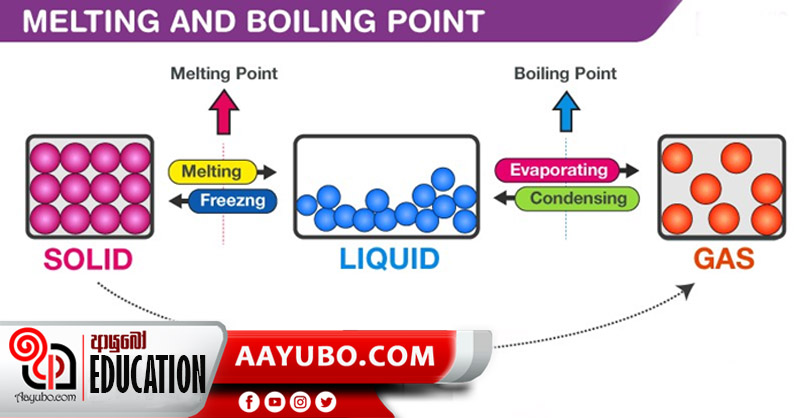
Properties like melting point, boiling point and density can be used in order to inspect the purity of matter.
Density is the amount of mass per unit volume of a substance. It is obtained by dividing the mass of a substance by its volume. Density of pure substances possesses a fixed/constant value where it helps to find the purity of substances that exist in all solid, liquid and gaseous states. Example: Density of water is 1000 kg m-3
Every pure substance has a constant/fixed temperature at which its state changes from solid to liquid and it can be cited as the Melting Point of that substance. This is normally called the melting point at normal atmospheric pressure where the temperature of that substance does not change as this conversion of state takes place. As the melting point of pure substances too are fixed, we can measure the melting point of a particular substance and check whether it’s pure or not. Example: The melting point of Ice is 00C .
The boiling point is the temperature at which a liquid turns in to gas (vapor). Pure substances have a fixed boiling point. This can be explained through a simple example. When we heat water, its temperature rises gradually where at a particular point, it stops and water turns in to water vapor from its initial state (liquid). Moreover, the boiling point of liquids relies on the atmospheric pressure. When the pressure falls, the boiling point also reduces and that is why the boiling point of water on top of a hill is less than the normal value; 100 0C . At the same time, if water is impure, the boiling point might take a value that is higher or lower than its normal value. Thus, this property can also be utilized to examine the purity of a substance. Eg: The boiling point of sulphur is 444 C.
by Mekhala Egodawele
Photo source : Internet
1016 Views







Comments